Purple Day
Eight fun ways to fundraise this Purple Day
Kami Kountcheva | Purple Day is less than a month away and there’s still time to grab a Purple Day Pack and get inspired by…
The latest epilepsy news
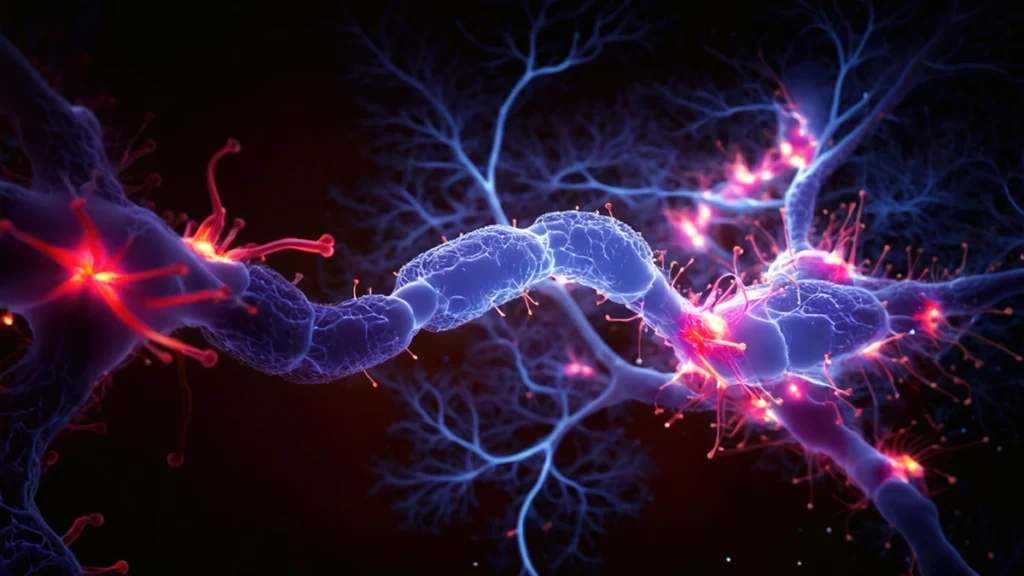
Zorevunersen “encouraging” new medication for Dravet syndrome, trial finds
Kami Kountcheva | A new study has found that zorevunersen is a promising new treatment for Dravet syndrome, a medication-resistant form of epilepsy
Drugwatch
Epilim Chronosphere (sodium valproate) modified release granules sugar free
The latest epilepsy news
Who is 2026 Miss GB Surrey contestant Emma Dearling?
Emily Stanley | With Epilepsy Action supporter Emma Dearling beginning a new venture as a contestant on Miss GB Surrey, we share six amazing facts…
The latest epilepsy news
Seven athletes with epilepsy and their stories
Kami Kountcheva | In celebration of the Winter Olympics coming to a close, we spotlight seven athletes with epilepsy and their epilepsy journeys
The latest epilepsy news
PIP assessments leaving claimants with “anxiety” and assessors quitting
Kami Kountcheva | The PIP assessment process is leaving people with epilepsy with “anxiety” and assessors feeling “despised”, while charities urge that the Timms review…
The latest epilepsy news
MedicAlert and Epilepsy Action renew partnership to support people living with epilepsy
Epilepsy Action and MedicAlert renew partnership to help bring peace of mind in an emergency to more people with epilepsy
The latest epilepsy news
Valproate scandal compensation: Wes Streeting commits to respond to Hughes Report before next election
Kami Kountcheva | The Health Secretary has said he will respond to recommendations in the Hughes Report into the valproate scandal before the next general…
The latest epilepsy news
Epilepsy on EastEnders: Is Nugget having seizures?
Kami Kountcheva | EastEnders is the latest soap to feature an epilepsy storyline, exploring different types of seizures and their impact on driving